,
Cder Guidance Calendar 2025
Cder Guidance Calendar 2025 – TEXT_1. TEXT_2.
Cder Guidance Calendar 2025
Source : www.raps.org
CDER 2024 Guidance Agenda New, Revised Draft and Immediately in
Source : www.fda.gov
FDA/CDER Readying Draft Guidance on AI to Support Regulatory
Source : www.pda.org
LDTs: FDA Rolls Out a Phased Implementation for New Regulatory
Source : www.foley.com
510(k) Third Party Review Program: FDA Offers New Draft Guidance
Source : www.raps.org
Half the Year Has Flown By – Plan Your Q1 2025 Exhibits Now!
Source : www.linkedin.com
FDA plans to release AI drug development guidance this year | RAPS
Source : www.raps.org
PDA/FDA Joint Regulatory Conference 2024 | PDA
Source : www.pda.org
FDA Issued a Final Guidance Document on CARES Act Reporting
Source : easconsultinggroup.com
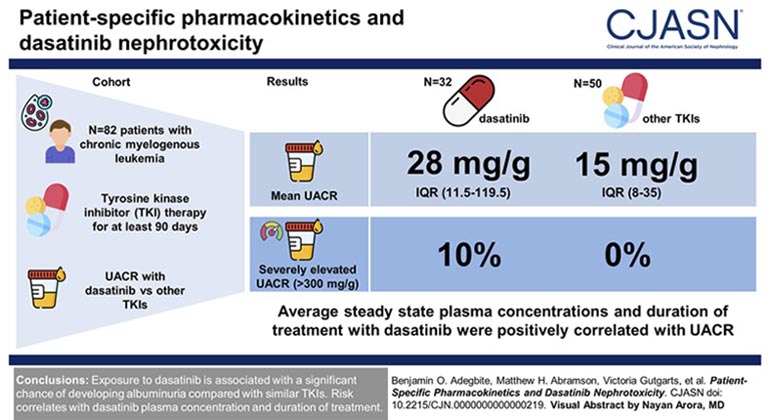
Investigators at Mount Sinai Find Strong Association of Kidney
Source : www.mountsinai.org
Cder Guidance Calendar 2025 CDER Guidance Agenda for 2019: What’s Coming | RAPS: TEXT_3. TEXT_4.
.jpg.aspx?width=350&height=434&ext=.jpg)